
Welcome to o g l c n a c .org
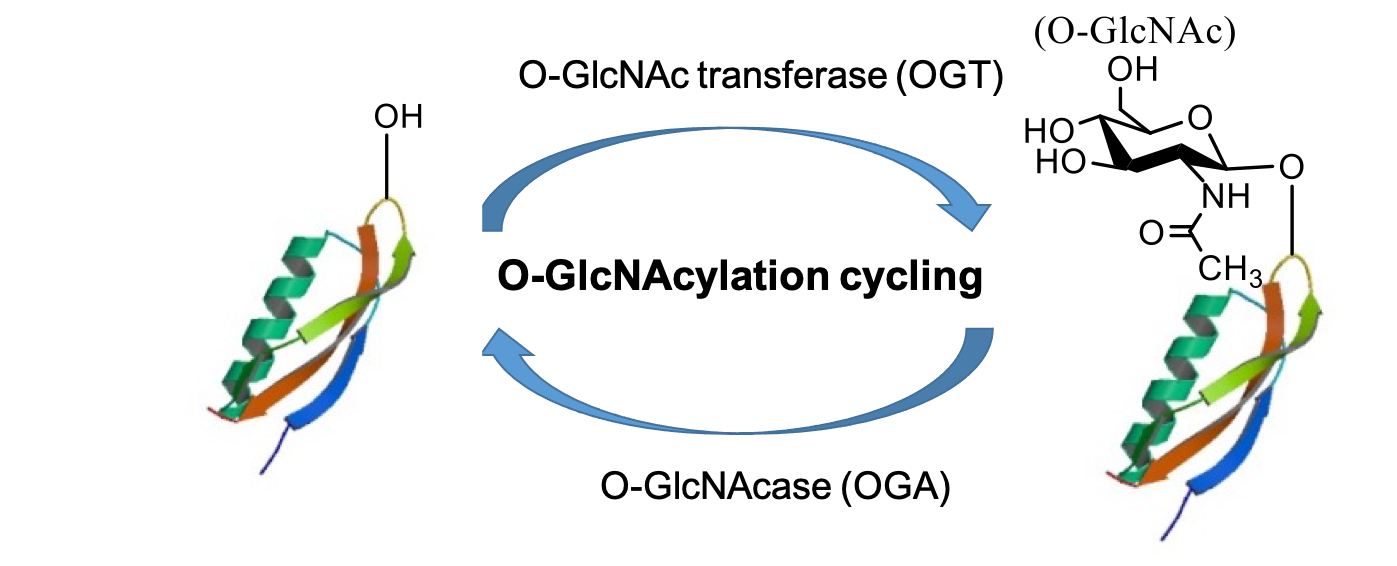
O-linked β-N-acetylglucosamine (O-GlcNAc) is a post-translational modification (i.e., O-GlcNAcylation) on serine/threonine/tyrosine residues of proteins. Distinct from the traditional glycosylation (i.e., N-glycosylation, O-glycosylation, and GPI-anchored glycosylation), O-GlcNAcylation is a unique intracellular monosaccharide modification without being further elongated into complex sugar structures. O-GlcNAcylation is catalyzed by a pair of enzymes: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). OGT transfers the GlcNAc moiety from UDP-GlcNAc to specific residues of target proteins while OGA removes it. As a highly dynamic modification, O-GlcNAcylation plays important roles in almost all biochemical processes examined (including transcription, translation, cell cycle, metabolism and signaling). Aberrant O-GlcNAcylation underlies the etiology and development of a number of chronic diseases (including cancer, diabetes, and neurodegenerative disease). Targeting protein O-GlcNAcylation holds great promise for biomedical applications (e.g., as therapeutic targets and biomarkers).
oglcnac.org currently maintains the following resources:
- O-GlcNAcAtlas, a rigorously curated database for experimentally identified O-GlcNAc sites/proteins.
- OGT-PIN, a rigorously curated database for experimentally identified interaction proteins of OGT and its orthologues.
- HexNAcQuest, a tool to distinguish O-GlcNAc and O-GalNAc.
- O-GlcNAcPRED-DL, O-GlcNAc site prediction with deep learning.
As a community server, oglcnac.org strives to provide scientifically solid and rigorous information for every piece of the content.
We welcome your feedback on how to improve it! Together let's make it better!